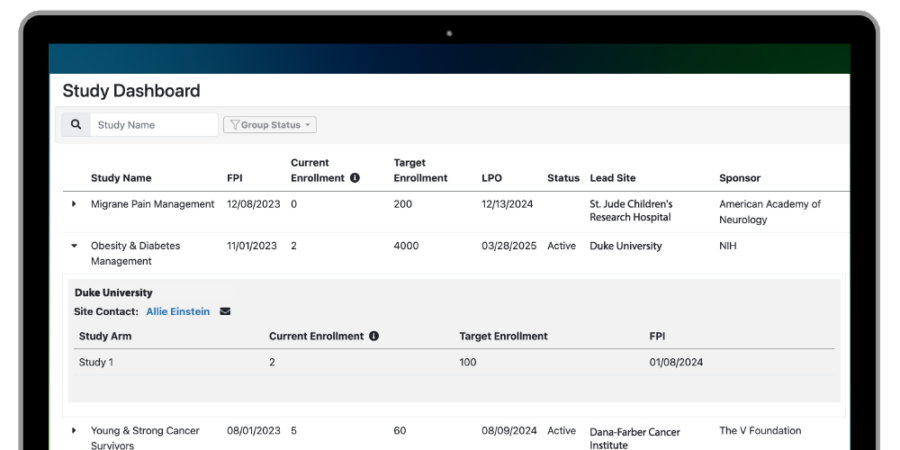
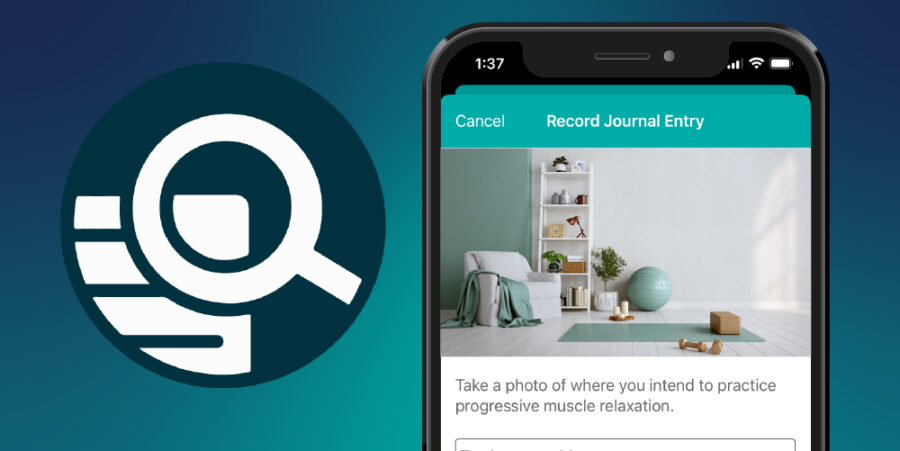
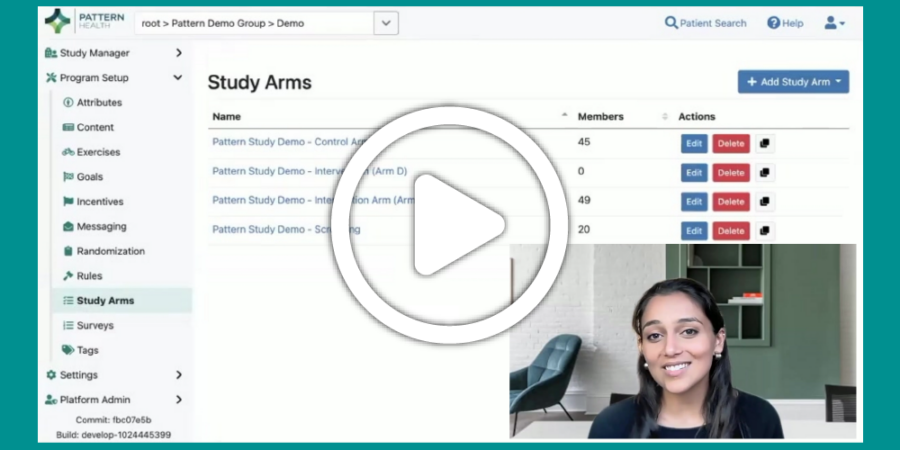
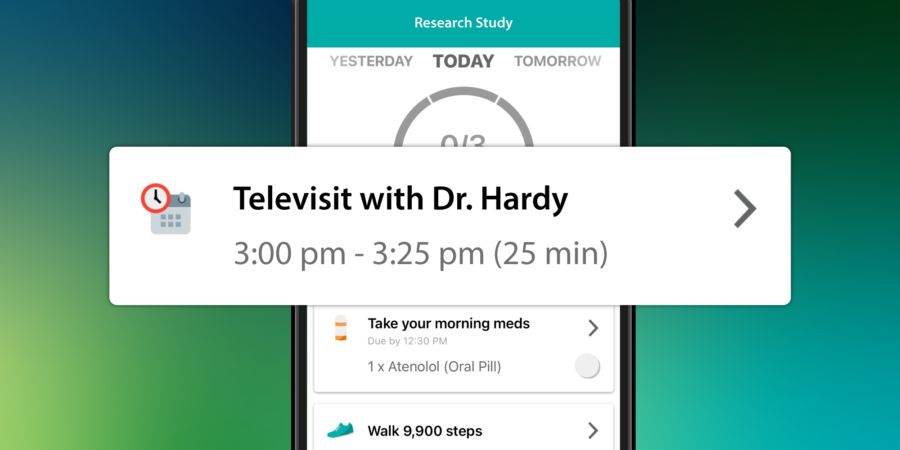
Clinical trial sponsors are constantly seeking ways to accelerate study timelines, thereby more rapidly delivering therapeutics to patients and optimizing return on R&D investment. Accelerating clinical trials means acquiring high-quality data faster. It means developing protocols, configuring technology, activating research...
ICAN-NC Team Partners with Pattern to Deliver and Study a Digital Health Intervention for Underserved Cancer Patients
The ICAN-NC study is evaluating the efficacy of remote therapy sessions and a supplemental app for breast cancer patients in rural areas. The digital health intervention, powered by Pattern, provides participants with symptom tracking and cognitive behavioral coping tools to help reduce pain, fatigue, and distress.
[Video] Integrating Biometric Devices with Pattern
Biometric data is a powerful addition to a study protocol, offering a variety of data about participants’ activity levels and well-being. Pattern has deep experience working with study teams to select the best-fit devices for their studies and integrating…
2023 Year in Review
As the year comes to a close, the Pattern Health team has taken some time to reflect. We are honored to support you, our amazing partners, who are pushing the boundaries of research and developing life-changing therapeutics. This holiday season,...
Integrated Televisits Now Available on Pattern Health’s Next-Gen Clinical Trial Platform
Pattern’s latest release enables a more streamlined experience for participants, providers, and research teams. The new functionality accommodates all varieties of virtual interactions, from remote eConsent visits to medical appointments.
IWQOL-Lite-CT©️ Implementation Options
IWQOL-Lite-CT© (Impact of Weight on Quality of Life-Lite-Clinical Trials) is the gold standard patient-reported outcome (PRO) measure of weight-related quality of life in adults designed for use in clinical trials. As the exclusive licensor of IWQOL-Lite-CT©, Pattern Health works with CROs and biopharma organizations to implement the measure for regulated clinical trials.
How to Choose the Best-Fit Biometric Devices for Research Studies
With the expanding market of biometric devices, selecting the right tools for research studies can be challenging. In this article, we provide guidance to healthcare researchers on selecting biometric devices, considering factors such as cost, provisioning process, battery life, user experience, device family, accuracy, and reliability.
EMPOWER Rare Disease Health Research Study Powered by Pattern Health
The Duke EMPOWER study team partnered with Pattern to help patients with Interstitial Lung Disease discover relevant clinical trials, access information and exercise plans, track biometrics, and more!
FDA Embraces Decentralized Clinical Trials (DCT): What You Need to Know
The U.S. Food and Drug Administration (FDA) recently released its long-awaited draft guidance supporting the use of decentralized clinical trials (DCTs) for drugs, biologics, and devices. The new guidance clarifies the FDA's position on DCTs, inspiring confidence in their use...
Pattern Health Launches Innovative Orthopaedic Risk Assessment Tool
Durham, NC, Nov 30, 2022 – Pattern Health today announced the latest addition to its digital health Exchange, Surgical Risk Prediction Suite (SRPS). SRPS is a clinical decision support tool authored by Duke Health that helps health systems anticipate and...
Heart Failure CONNECT-HF Study Used Pattern Health App to Study Medication Adherence, Quality-of-Care Scores
The Duke Clinical Research Institute study was the first to incorporate mobile health technology to follow heart failure patients long-term. The study showed that using patients’ biometric information and medication adherence data can help customize guideline-based recommendations to improve self-care....
Pattern Health Closes $3.3M Series A to Expand Digital Health Research and Clinical Implementation Capabilities
Durham, NC, Sep 22, 2022 – Pattern Health, a digital health company, today announced the closing of a $3.3 million series A funding round. The round was led by the Dr. William H. Joyce Family Office, with contributions from existing...