
The good news is that remote research has been successfully conducted for years, and there are many ways to adapt to the new policies. Better still, the NIH is providing funding support to adapt research projects to utilize remote or “virtual” participant interactions and data collection. And once researchers experience the many benefits of virtual studies — convenient, efficient, unconstrained geographically — they may not wish to turn back.
Pattern Health’s digital health platform powers research studies and clinical trials in which people participate from the comfort of their home, during work, and as part of their daily routine. In fact, ten of the top 20 academic medical centers are already using Pattern Health to conduct research and real-world evidence (RWE) studies. The platform lets researchers onboard and obtain informed consent from participants, manage participation throughout the study, collect data and administer activities — all remotely. Because the platform can be configured quickly, adapting to remote research need not delay study timelines.
Here’s how it works
Participant recruitment
Participants may be recruited remotely from a variety of sources that are easily linked to the Pattern Health platform.
-
Electronic health record (EHR) databases (contact by phone, email, or other digital medium)
-
Online forums and groups
-
Advertising – all types – search, print, local, targeted digital
-
Online population sampling tools like Prime Panels
-
Telehealth visits
Studies that were previously recruiting at the point of care may still be able to reach the same population remotely. The best source of participants for your research study depends on your access to the source and your eligibility criteria. For example, if your eligibility criteria is a diagnosis or status that persists over time (chronic conditions, pregnancy, age group, weight, tobacco use, prescribed medications, procedure, etc) and you have authorization to sample from a provider’s patient population, an EHR database may be an ideal option because you can search the database for potentially eligible participants. If your eligibility criteria involve short-term symptoms (fever, etc), EHR database information may not be sufficiently up to date, so telehealth visits or targeted digital advertising may be better options.
Eligibility, informed consent, enrollment, and onboarding
Participant eligibility, informed consent, enrollment and onboarding can all be conducted remotely using the digital tools in the Pattern Health web and mobile applications. Potential participants can complete personalized study screening questionnaires. Then, eligible participants can learn about the study and electronically sign consent agreements, while collecting all necessary documentation and signatures. Participants may be automatically allocated to a study arm based on randomization criteria and participant attributes, and immediately enrolled and onboarded to a dynamic program that they can participate in from home.
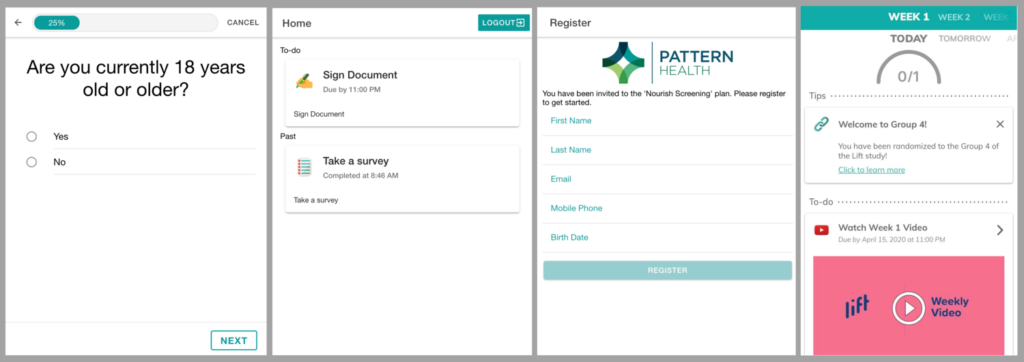
Intervention delivery
Studies have a wide range of options for delivering interventions remotely. The Pattern Health platform comes with a comprehensive set of evidence-based engagement and behavior change features including scheduled activities and digital content delivery with tailored reminders; group and individual communication over video, phone, and chat/text; implementation intentions; virtual pets and much more. For interventions that have no digital alternative, such as drugs or medical devices, postal, package and special delivery services may in some cases be an option to replace in-person administration — yes, Pattern Health helps manage this too.
Data collection
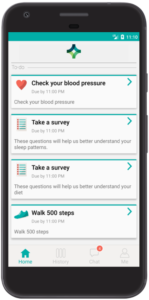
Every research study needs to collect data, whether biometric data, behavioral data, patient reported outcomes (PROs) or other real-world data (RWD), and the Pattern Health platform provides a comprehensive set of tools to collect many different kinds of data remotely. Biometric data, behavior and activities can be collected from home devices and wearable sensors, often automatically and passively, with manual data entry via the mobile or web application serving as a reliable backup option. Digital surveys and self-report assessments enable remote collection of self-reported measurements and outcomes (PROs). Assessments can adapt dynamically and be scored in real-time. Researchers can create their own custom assessments or select from a library of validated instruments. Assessments and all content in the Pattern Health platform can be translated and delivered in the participant’s preferred language. Virtual study visits via video, phone or chat/text communication enable interactive data collection by study coordinators.
Participant management
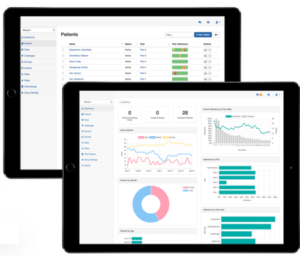
Researchers (and if appropriate, participating providers) can track participant engagement, adherence, data, messages and status using the Pattern Console, the platform’s application for research administrators. The dashboard and reporting tools help easily identify and prioritize check-in communication or other interventions. Researchers can also configure rules to trigger automatic, data-driven actions.
Translating research into practice and the real world
Ensure that impactful discoveries can be easily translated into real-world programs by choosing technology that supports both research and the real world, which is one of the unique advantages of the Pattern Health platform. If desired, program creators can also make their programs available for others to license through the Pattern Health Marketplace.
Beyond COVID-19
The COVID-19 health emergency is forcing us all to act and think in new ways, to adjust to new constraints. But there are lessons we can carry forward even once restrictions are lifted. Remote research presents an opportunity to improve geographic diversity, participant and researcher convenience, and to run more cost-efficient research studies. For many studies, these advantages will continue to apply even when in-person interactions are safe again.
Need help transitioning your research study to digital?
Get in touch with us here.